Big Red Machine: Cardiovascular Engineering at Cornell
By Syl Kacapyr
After experiencing some mild chest pain, you step into your physician’s office for a diagnosis. Your physician uses a laser to observe the cells in your heart and discovers some damaged muscle. A computer analyzes your patient file and quickly offers a menu of solutions, each with a percentage indicating the likelihood the procedure will work. You select the computer’s recommendation to have your heart locally injected with specialized molecules to regenerate the tissue, and your physician will 3D print a new valve made of your own living cells and in the shape you need.
While such an experience remains science fiction, research underway at Cornell Engineering is offering a glimpse into the future of cardiovascular care. New tools and techniques promise to change the way heart disease is treated and prevented, and they’re being developed in the face of a stark reality: Over 92 million Americans are living with some form of heart disease or the after-effects of stroke, according to the American Heart Association. Heart disease continues to be the leading cause of death worldwide, contributing to one in every three deaths in the U.S.
Much of this research at Cornell is happening in the Nancy E. and Peter C. Meinig School of Biomedical Engineering, which has added two prominent engineers to its faculty, establishing a critical intellectual presence in cardiac technology. The school is also finding new ways to capitalize on partnerships and collaborations around campus, pushing the limits of imagination and breaking the rules to advance cardiovascular engineering.
Heart-Assist Devices
When James Antaki was 11-years-old, he was determined to repair an old, antique radio sitting in his parents’ basement. He bought an electrical engineering book and taught himself just enough to get the radio working. Years later, Antaki would find himself as a graduate student at the University of Pittsburgh, still repairing broken machines, but this time it would be an artificial heart that was being used by a new university program.
It was there Antaki—who will join the Meinig School in January as the Susan K. McAdam Professor of Heart-Assist Technology—pioneered a magnetically levitated rotodynamic heart-assist device, a blood pump dubbed the Streamliner, that became the first of its kind to reach pre-clinical testing.
Like other ventricular devices that came before it, the Streamliner was designed to attach to a patient’s heart, helping to pump blood from a weak left ventricle to the aorta. But unlike the first heart-assist devices approved by the FDA in 1994, Antaki’s device ditched the unreliable bearings used to suspend the pump’s impeller, and replaced it with a magnetically levitating one. He continued to engineer several iterations of the device, including PediaFlow, a prototype heart-assist device for infants.

“Currently, the pediatric physicians have no options other than a giant refrigerator-size pump that has more blood outside of the baby than inside,” explains Antaki, “and they have horrible incidents of stroke and bleeding. Survival is very poor.”
Today, magnetic levitation pumps are now at the forefront of ventricular heart-assist devices, but the technologically-advanced Streamliner is not one of them. Neither is the PediaFlow. While Antaki helped pioneer modern heart-assist devices, intellectual property rights and business decisions that were out of Antaki’s hands stifled his ability to bring tech advancements to the market.
Antaki views Cornell as a fresh start. He’s in the process of recapturing his patent rights, and points to the words “heart-assist technology” in his title as an example of how Cornell’s priorities are aligned with his. He says one of the biggest draws to Cornell was the opportunity for research collaboration.
“Cornell’s world-class veterinary school is a huge asset,” says Antaki. “Having it in my backyard versus having to fly to the West Coast to do experiments—it goes without saying that it will accelerate our pre-clinical testing.”
Biomaterials
Pediatric cardiology was thrust into the national spotlight in May of 2017 following an emotional episode of the late-night talk show “Jimmy Kimmel Live.” In the episode’s opening monologue, host Jimmy Kimmel, fighting back tears, revealed that his newborn son had just undergone emergency open-heart surgery after he was diagnosed with a rare congenital defect in which a hole existed between his heart’s bottom two ventricles.
Surgeons at Children’s Hospital Los Angeles were able to sew a biomaterial over the hole, “just like you would sew a patch onto a pair of pants,” one surgeon told the Los Angeles Times. He noted that Kimmel’s son would need that patch replaced as his heart grows larger.
“Hopefully someday we’ll develop materials that will grow and expand over time,” stated the surgeon. Cornell engineers are doing just that, engineering tissue in the form of living prosthetics.
After watching his mechanical engineering colleagues pioneer a method for 3D printing cartilage in the form of vertebral discs, menisci and even ears, Jonathan Butcher wondered if it would be possible to use the same process to print heart valves. So Butcher, an associate professor and associate director of the Meinig School, began an ambitious project to create an anatomically correct, mechanically heterogeneous, living heart valve with a 3D printer.
The project required the development of cell-friendly hydrogel materials that could be deposited by a 3D printer and mimic the unique physiological biomechanics of different valve structures. It also required developing a system to cure the deposited material while it was being printed, and computer algorithms to process clinical 3D-image datasets to identify not only the precise valve geometry, but also the different internal material domains within it.
“Over the past 10 years, we have developed the most complex and realistic living human-sized heart valve from scratch. These achievements have the potential to overcome many of the limitations of current tissue-engineered valve technology, and hopefully provide the key needs of biointegration, growth and life-long function,” says Butcher.
While non-living prosthetic valves perform well for elderly patients, the same isn’t true for younger patients with congenitally malformed valves. The need for a better alternative is compounded when one considers the millions of young patients suffering from valve disease in the developing world, up to 10 percent of hospital admissions in some communities, according to Butcher.
 But proving life-saving technology is safe and effective in children requires a high regulatory burden, including animal and clinical trials. Butcher hopes promising results in large animal models will spur further investment in the research.
But proving life-saving technology is safe and effective in children requires a high regulatory burden, including animal and clinical trials. Butcher hopes promising results in large animal models will spur further investment in the research.
“It is a tough ‘dead zone’ where academic funding often ends, but industry is not yet willing to pick it up,” he says.
To bridge the gap, Butcher is developing a more near-term solution: a bio-hybrid prosthetic valve that also works as a scaffold to hold living cells. The patented device is designed to help the valve regulate normal function within the heart, such as coagulation and protein absorption. He plans to work with Antaki on preclinical testing.
Foam Heart
With both arms stretched out, Ben MacMurray uses latex gloves to gently hold what looks like a human heart in the palms of his hands. Without warning, the Ph.D. student squeezes the heart, but no blood comes out. Instead, the heart quickly expands back to its original shape, as if MacMurray had just gripped a Nerf football.
The object is a prototype prosthetic heart, engineered using a lightweight, stretchable, poroelastic silicone material in the lab of Rob Shepherd, associate professor of mechanical and aerospace engineering. Shepherd’s lab often experiments with new materials for use in ‘soft’ robots that are actuated using compressed air, but when it came time to demonstrate the poroelastic silicone, Shepherd had a different idea.
“We decided to use a heart as an example because it’s a very complex shape and it’s a machine that everyone’s familiar with.” says Shepherd. “We thought it would demonstrate our material’s capability the best. It turns out, people are very interested in using it as a heart replacement or even an assistant machine to a heart.”
The porous material allows fluids to pump through much like the way a real heart would, and because the prosthetic heart is engineered using a single material, it’s a simple machine with fewer opportunities for failure.
It’s a novel idea, and one that has received some attention as wait lists for heart transplants remain long. Nearly 4,000 U.S. patients remain on the heart transplant list at any given time, with an average of 18 people dying each day as they wait, according to the American Heart Association.
Shepherd and his team are now working with medical doctors at Weill Cornell Medicine to turn the material into a useful medical device.
Regeneration
One of the unique powers of the comic book superhero Wolverine is that he has the ability to regrow parts of his limbs and organs after injury. It’s a remarkable gift that, in real life, is difficult-to-impossible for most mammals to replicate, depending on the organ.
That is partly why heart disease is so deadly for humans. Heart attacks occur after blood flow has been reduced to an area of the heart, causing that area of tissue to be damaged or dead. Because the heart doesn’t have the ability to regenerate, the damage becomes permanent and the victim is left with a weaker heart.
But other parts of the animal kingdom do exhibit the superpower of regeneration. Amazingly, the zebrafish can lose up to 20 percent of its heart before regrowing the tissue in just a matter of days. This piqued the curiosity of Yadong Wang, the McAdam Family Foundation Professor of Heart-Assist Technology in the Meinig School, who has been engineering new biomaterials, some of which are used for regenerative medicine.
“Being a chemist by training, I’ve always been really interested in making new things, but mother nature has been doing that for millions of years,” says Wang, referring to the zebrafish’s ability to regenerate its heart. Part of the secret is in the zebrafish’s extracellular matrix (ECM), essentially a collection of molecules that provide structure and biochemical support to surrounding cells. It’s this ECM that works to repair the heart, although scientists don’t understand exactly how.

Still, Wang wanted to know how zebrafish ECM would perform in a mammalian heart, so he injected some directly into mice that had suffered a heart attack. What he discovered was the ECM worked to protect myocytes, the specialized cells that make up heart muscle, and within five days he observed an improvement in heart performance in many of the mice. While less than 1 percent of the myocyte was affected, Wang says it’s enough to demand more research.
“It’s a start. There’s something there,” he says.
Scaffolds
Wang has also engineered grafts and scaffolds that not only serve as an artificial component of a heart, but also attract and activate cells that can help the heart regenerate.
“We need to design the signals in our scaffold so the immune system recognizes it as a relatively benign material,” says Wang, adding that the wrong type of material could be rejected too quickly by the recipient’s body. “While the immune system is slowly degrading this material, the cells are honing in and making tissue.”
But recruiting the cells is only the first step. The biomaterial the scaffold is made of must also activate the cells to perform the correct tasks. The trick is getting monocytes, a type of white blood cell, that encounter the biomaterial to differentiate into the proper mix of macrophages, cells that will destroy any proteins it considers to be harmful to the body.
Wang has discovered a number of materials that don’t negatively interact with monocytes. The most promising, he says, is a biodegradable elastomer material he has nicknamed “biorubber.” It looks like silicone, but is actually a poly-gylcerol sebacate that has successfully been implanted in animals to help them regenerate bone, cartilage, blood vessels and heart tissue.
 Wang, who just began his first year at Cornell, says “I haven’t been to a university that has a veterinary school.” He expects the ability to collaborate with the College of Veterinary Medicine to accelerate his research and allow him to have a better view of how his biomaterials are performing. He also plans to revisit his roots in chemistry through his new colleagues in the Robert F. Smith School of Chemical and Biomolecular Engineering and The Department of Chemistry and Chemical Biology.”
Wang, who just began his first year at Cornell, says “I haven’t been to a university that has a veterinary school.” He expects the ability to collaborate with the College of Veterinary Medicine to accelerate his research and allow him to have a better view of how his biomaterials are performing. He also plans to revisit his roots in chemistry through his new colleagues in the Robert F. Smith School of Chemical and Biomolecular Engineering and The Department of Chemistry and Chemical Biology.”
Computation
As part of the University of Pittsburgh team experimenting with heart-assist devices, Antaki encountered a problem that threatened to jeopardize the entire program. It was the mid 80s and the blood inside the valve of an implanted heart pump clotted. Little was understood about the underlying cause of how and why blood clotted inside prosthetic organs, and so Antaki became determined to develop a computational model that could explain.
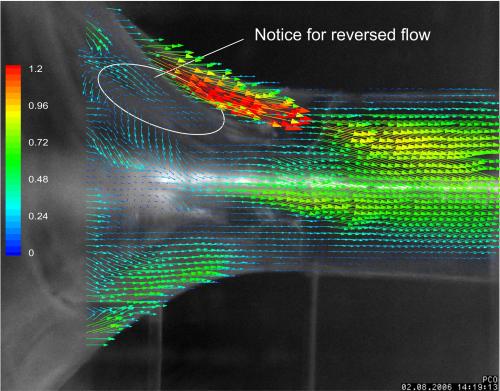
Years later, Antaki says “we now have a computer simulation that works remarkably well under certain conditions.” His model worked well enough to earn a National Institutes of Health grant for the next four years to attempt to predict circumstances under which blood will clot in heart-assist devices.
Modeling something as complex as blood flow is no easy task, but the benefits are numerous. Not only can the data help engineer better ventricular devices, but it can simulate risks for individual patients and help manage their post-surgery recovery. For example, Antaki’s statistical model aims to advise patients on whether or not they should get a device, and if so, which one. It can also dictate how patients should manage their diet to reduce the risk of stroke, bleeding and other adverse consequences of receiving an implant.
When it comes to heart disease, computation is showing great promise in its application to precision medicine as well. When used in conjunction with the human genome, it can be a powerful tool to predict the most effective medication for an individual patient, and to identify which risk factors need to be considered most aggressively.
“There have been some recent studies that have used large genomic data sets to begin to get predictive algorithms for patients that are more likely to develop coronary artery disease or atherosclerosis. And in particular, which patients will benefit from taking one of the statin medications that lower cholesterol,” says Geoffrey Pitt, director of the Cardiovascular Research Institute at Weill Cornell Medicine. “There is a separate effort in looking at trying to predict patients who will be subject to atrial fibrillation, which is an arrhythmia that’s much more common in elderly patients.”
This type of precision medicine has not been as prevalent for predicting and treating heart disease as it has been for cancer, but it’s an initiative Pitt plans to bring to the forefront through his institute, which launched in 2016 with the idea of building a larger cardiovascular research program that combines Weill Cornell Medicine’s outstanding clinical care with its growing research infrastructure.
Pitt, who has collaborated with Butcher on several research grants, hopes to use the institute to encourage more collaboration, especially with the Ithaca campus and Cornell Tech, both of which provide leading expertise in engineering the types of algorithms needed to analyze large sets of genomic data.
Imaging
Another priority area for the Cardiovascular Research Institute at Weill Cornell Medicine is to understand the way small molecules interact with larger biological systems in cardiovascular disease. Luckily, the institute has to look no further than their engineering colleagues in Ithaca for some of the most advanced imaging tools and techniques that science has to offer.
Recently, a team of Cornell researchers imaged, with single-cell resolution, an in-vivo beating heart. The team was led by Nozomi Nishimura, assistant professor of biomedical engineering, who says looking at how individual cells function inside a live, beating heart has been a major challenge for scientists.
“The main problem is that the heart is moving while it is pumping blood and so it’s actually been very difficult to get a picture of the underlying cellular behavior,” says Nishimura. “The heart is a very dense, blood rich tissue and it’s very opaque. So to study blood flow inside the heart wall you really need previously unattainable depth of imaging.”
Nishimura was able to achieve the unprecedented microscopic view using three-photon microscopy, a technique developed in Cornell’s Department of Applied and Engineering Physics that uses high-intensity lasers to non-invasively excite and image at a point of interest, such as a single cell. Originally engineered with neuroimaging in mind, Nishimura adapted the microscope for a mouse heart by speeding the imaging rate.
“We’re finally at the point where we can reliably image how the many aspects of heart physiology work together. These include blood flow, cardiomyocyte action potentials, as well the immune and the inflammatory cells within the living heart,” says Nishimura, noting the imaging technique will be important for understanding how heart attacks propagate and the cellular basis behind heart failure.
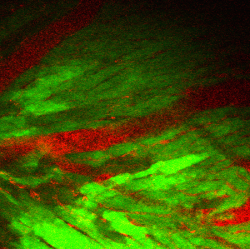 Nishimura’s lab is using lasers not just for imaging cells, but also for manipulating them. Imagine a scalpel not only capable of slicing off a small portion of a cell, but doing so inside a living animal without having to touch any other part of its body. This is what Nishimura has achieved using a powerful femtosecond laser that blasts photons at its target using pulses that flicker at mere millionths-of-billionths of a second. Using this technique, Nishimura can induce small blood clots or hemorrhages to mimic aspects of cardiovascular disease.
Nishimura’s lab is using lasers not just for imaging cells, but also for manipulating them. Imagine a scalpel not only capable of slicing off a small portion of a cell, but doing so inside a living animal without having to touch any other part of its body. This is what Nishimura has achieved using a powerful femtosecond laser that blasts photons at its target using pulses that flicker at mere millionths-of-billionths of a second. Using this technique, Nishimura can induce small blood clots or hemorrhages to mimic aspects of cardiovascular disease.
Nishimura has been able to advance her work though an ongoing collaboration with Provost Michael Kotlikoff’s research lab in the College of Veterinary Medicine, which has engineered calcium-sensitive proteins needed to make her imaging techniques work. Her lab is also developing optogenetic tools to stimulate the electric activity of hearts.
“We basically get first crack at trying some of these new tools, so this is a very exciting collaboration,” says Nishimura.
The Future
Emerging trends and technologies in cardiovascular research are providing new hope in the fight against heart disease. The addition of Antaki and Wang to Cornell’s talented faculty expands its intellectual footprint and uniquely positions the university to be a leader in cardiac technology.
“It's an exhilarating time for cardiovascular research at Cornell," said Marjolein van der Meulen, director of the Meinig School. “We are fortunate to have attracted these two senior leaders who complement our existing strengths, and I look forward to the opportunities and synergies created as these groups build new connections and collaborate with physicians and industry.”
A Conversation with Cornell Engineer Rob Shepherd
“We decided to use a heart as an example because it’s a very complex shape and it’s a machine that everyone’s familiar with.” says Shepherd. “We thought it would demonstrate our material’s capability the best. It turns out, people are very interested in using it as a heart replacement or even an assistant machine to a heart.”

