Inside the tumor microenvironment
By Chris Woolston
Pulling together techniques and insights that span multiple scientific fields and academic departments, researchers from Cornell Engineering and Weill Cornell Medicine are taking an expansive look at cancer that goes beyond tumors and individual cells. Bioengineers, chemists and cancer biologists are teaming up to understand the physical conditions surrounding each cancer cell, also known as the tumor microenvironment. By exploring the nuances of the human body from cancer’s point of view, they’re hoping to uncover weaknesses and vulnerabilities of the disease that can inspire new approaches to treatment and prevention.
“Cancer isn’t just about the tumor cells themselves,” says Claudia Fischbach-Teschl, professor of biomedical engineering and director of Cornell’s Physical Sciences Oncology Center on the Physics of Cancer Metabolism. “If we understand how the microenvironment might be driving tumor development and expression, we could try to interfere.”
The effort is unfolding on multiple fronts. Fischbach-Teschl and Lewis Cantley, the Meyer Director of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine, are co-principal investigators on a project that’s exploring the effects of the microenvironment on the metabolism and energy supply of tumors. The project is part of the Physical Sciences-Oncology Network, a five-year National Cancer Institute initiative that enlisted top cancer centers from around the country. “We have the expertise to address issues that classic cancer centers can’t address,” Cantley says.
The obesity factor
As part of the nationwide initiative, Cornell scientists are taking a close look at the ways that obesity shapes the microenvironment around tumors. Researchers have long known that extra weight seems to encourage the growth and spread of several cancers—notably breast cancer and endometrial cancer—but the root causes behind the link have been unclear. “Obesity might be priming the microenvironment in a way that allows cells to quickly become cancerous,” Fischbach-Teschl says.

Research so far has identified some intriguing connections between excess weight and the microenvironment for cancer. Obesity can increase inflammation in the tumor microenvironment, which in turn can help prevent immune system T-cells from reaching and perhaps killing rogue cells. As Fischbach-Teschl and Andrew Dannenberg, Henry R. Erle MD-Roberts Family Professor of Medicine at Weill Cornell Medicine, describe in a 2019 issue of American Journal of Pathology, obesity can also stiffen the immediate surroundings of a cell—called the extracellular matrix—by boosting levels of collagen and other structural proteins, and these changes can affect immune cells. Cancer cells stiffen the matrix in a similar way when given the opportunity, suggesting that any rigidity related to obesity helps makes the microenvironment more hospitable to cancer survival and growth.
Cantley and his lab are especially interested in the connection between obesity, cancer and PI3-Kinase, an insulin-regulating enzyme that Cantley first discovered in the early 1980s. Over the years, researchers have found that cancer cells in the breast and endometrium carry a heavy load of mutated versions of the enzyme, alterations that make them especially sensitive to insulin. Obese people tend to have high levels of insulin, and the hormone likely helps fuel the growth and spread of cancer cells that carry mutations to PI3-Kinase. “We’re figuring out how insulin signaling plays a role in cancer,” Cantley says.
Engineering meets biology
To better understand how cancer cells react to their surroundings, Cornell researchers have gone to great lengths to recreate the biological microenvironment where cells grow, a challenge that has inspired new feats of bioengineering. Fischbach-Teschl works closely with Lara Estroff, a professor of materials science and engineering, to produce artificial scaffolding that mimics the structure of human bone.
Cells growing on a flat plastic plate may be easier to track and observe, but Fischbach-Teschl notes that flat plastic is a poor model for real-life growth. “Scaffolds allow cells to grow in a three dimensional context,” Fischbach-Teschl says. “It’s almost like they are making their own microenvironment and doing their own thing.” The scaffolds, created with a polymer called poly(lactide-co-glycolide), are about the size of an aspirin tablet and are so full of pores and open spaces that they’re nearly as light as air.
The scaffolds may be insubstantial, but the implications of the research are enormous. About 70% of all cases of metastatic breast cancer involve cancer of the bone. At the same time, about 70% to 80% of breast cancers diagnosed at an early stage when they are still confined to the breast never go on to become metastatic. Fischbach-Teschl and others suspect that the microenvironment around the cancer cells plays a major role in determining which cells turn cancerous and which cancers will eventually become aggressive and potentially deadly.
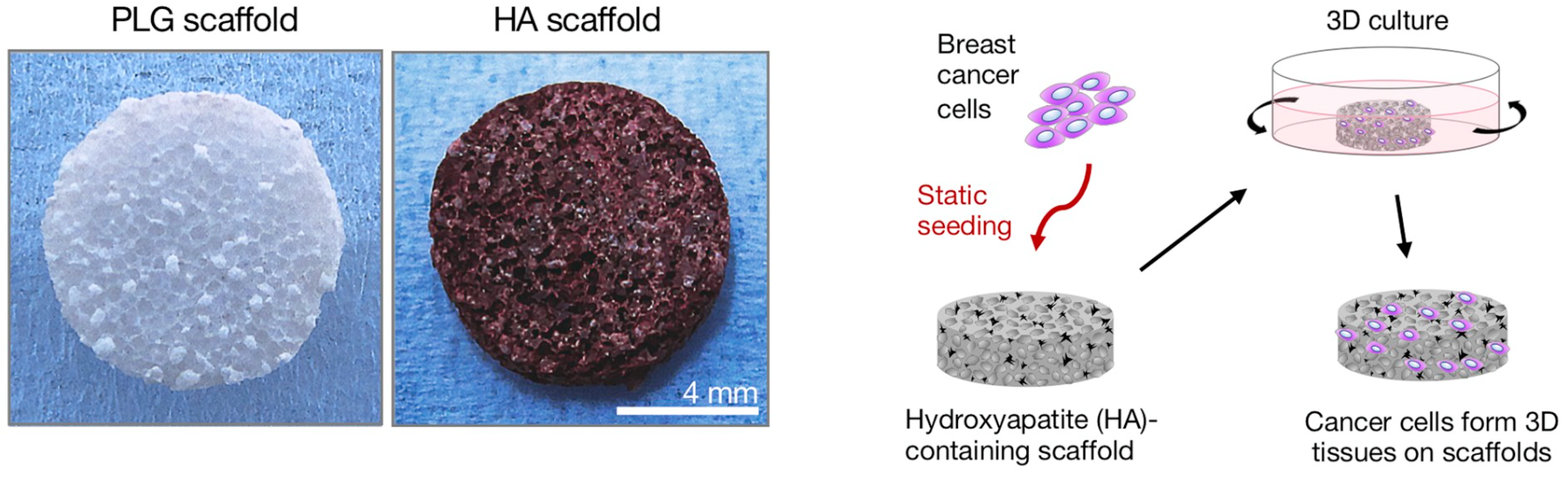
As Fischbach-Teschl, Estroff and a team of 16 co-authors reported in the December 2019 issue of Biomaterials, subtle changes in the bone environment can have a big effect on cancer cells. The researchers found that lacing the scaffolds with hydroxyapatite, a natural mineral often found in cancer-ridden bone, encouraged aggressive behavior in human breast cancer cells. It’s not clear how the mineral accomplishes that trick, but Fischbach-Teschl notes that hydroxyapatite is a very “sticky” mineral that often glues itself to proteins. Proteins that stick to the mineral may become altered in their molecular structure, potentially changing the way cells interact with them. These altered interactions could ultimately lead to differences in tumor cell growth and invasion.
The interplay between cancer and the microenvironment is clearly a two-way street. Cancer cells respond to their immediate surroundings, but they also shape that environment to their advantage. Fischbach-Teschl explains that metastatic breast cancer can degrade bone in much the same way as the bone-thinning disease osteoporosis. As cancer cells chip away, the bones release growth factors that spur the cancer to grow even faster. Fischbach-Teschl and other researchers are currently using scaffold models to learn more about the ways that cancer remodels bone cells and search for ways to short-circuit the process. “If you can slow down or stop the degradation of the bone, you can slow down tumor growth,” she says.
Tight spaces
The movement of cancer cells through their environment is also a major research focus for Jan Lammerding, an associate professor at the Meinig School of Biomedical Engineering. Mobility is a crucial component of cancer, he says, because 80% of all cancer deaths are caused by metastasis, when tumor cells spread to distant organs. Lammerding tracks cells as they squeeze through tiny passages in a microfluidic device that’s precisely engineered to replicate the small pores and channels in the human body. “The device mimics what a cell would find, whether it’s a breast cancer cell moving through a dense matrix or another cancer cell squeezing through the walls of a blood vessel,” he says. While building the device, Lammerding and his team were able to enhance the realism by consulting images of actual tumor cells moving in living mice. The images were created by Peter Friedl, professor of medicine at the University of Texas’s MD Anderson Cancer Center in Houston, who is also part of the Cornell Physical Sciences Oncology Center.
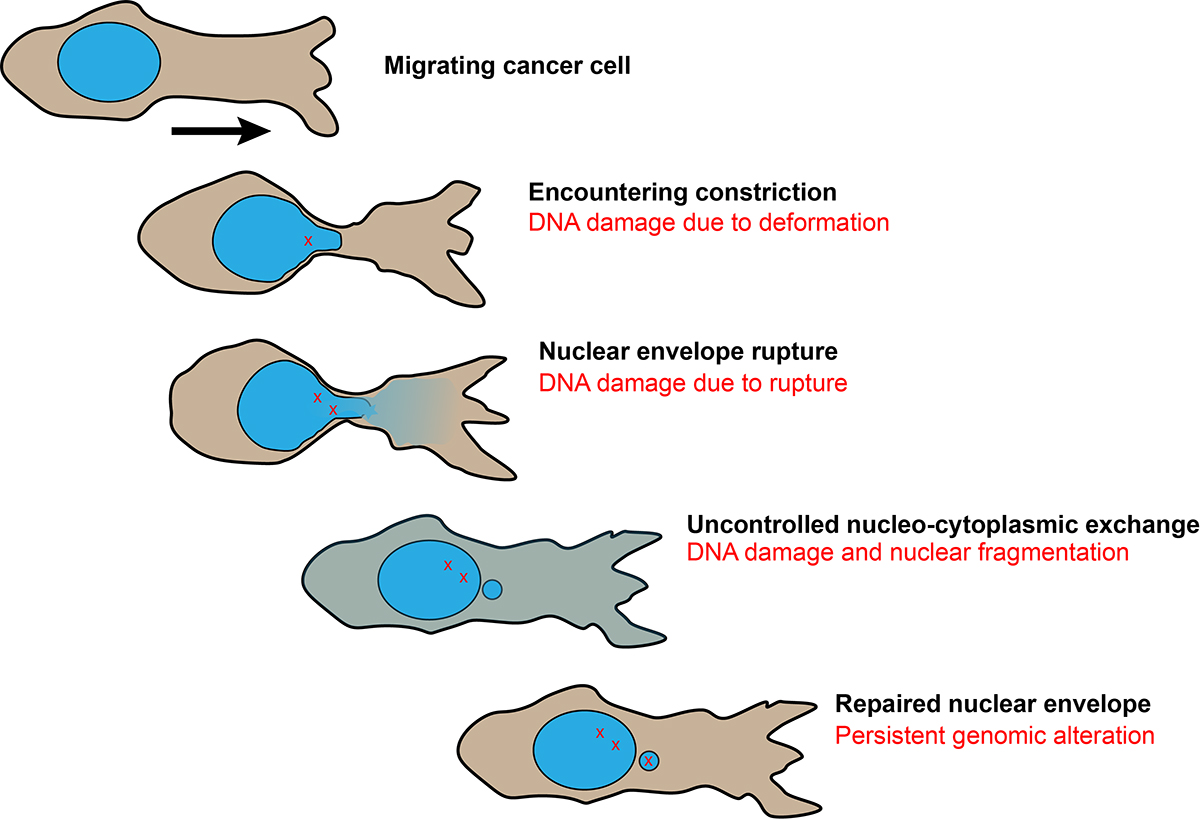
The journey of a cancer cell can be perilous—for both the cell and the patient. In a paper published in the journal Science in 2016, Lammerding and colleagues found that nuclei can become stressed and rupture while squeezing through tight passages, potentially damaging DNA in the nucleus. As a result, cells that survive the process may become more aggressive and resistant to therapy.
In ongoing research, Lammerding is looking at the proteins called lamin A/C that help stiffen the cell nucleus, making it more difficult for cells to fit through tight spots and travel through the body. Work with cultured tumor cells shows that low levels of lamin A/C facilitate cell migration through small pores, and analysis of tumor biopsies from breast cancer patients shows that patients with low levels of the proteins in their tumor cells tended to have a larger risk of cancer metastasizing or recurring. “We’re trying to understand the difference between cells that are likely to metastasize and those that aren’t,” he says.
Cancer cells need energy as they move through their microenvironment, a requirement that researchers are hoping to better understand and potentially exploit. Using biosensors developed together with Warren Zipfel, an associate professor at the Meinig School of Biomedical Engineering, Lammerding can track the energy usage of cells as they travel through the device. “Some fast moving cells may also have high energy requirements,” Lammerding says. “We can test different drugs to see if they inhibit energy production and cell mobility. It’s something that we’re very excited about.”
Lammerding says his collaborations with Zipfel and others underscore the cooperation and synergy needed to understand the complicated world of a tumor microenvironment, a world that combines elements of physics, chemistry, engineering and biology. “It’s a wonderful area for interdisciplinary work,” Lammerding says. “By bringing in engineers and combining cell biology with microfabrication, we can do things that no individual group would be able to do.”
All of this work on the microenvironment is part of a bigger vision: Understanding cancer well enough to keep it under control. “Finding a cure for cancer may be unrealistic in many cases,” Fischbach-Teschl says. “But we can do much better to make it a manageable disease.”

